
Introduction to Low-Level Laser Therapy (“LLLT”) Technology
Medical researchers began using laser biostimulation in the late 1960’s with low-
powered laser beams that produced non-thermal effects on human tissue. The first
reported cases involved slow-healing ulcers. The efficacy of this low-level laser
therapy, or “LLLT,” is substantiated by objective research that continues.
Experimenting clinicians found that an 830 nm laser is optimal for treating chronic
pain. An example of how LLLT works involves soft tissue trauma. These types of
injuries consist of damage to the deep, sensitive layers of tissue beneath the
epidermis, including muscular, neural, lymphatic, and vascular tissue
The human body normally reacts to this soft tissue trauma by “splinting” the injury
with edema, a thin or watery fluid in tissue spaces or cell interstices. However,
excess edema causes swelling that inhibits movement of the damaged tissue. These
injuries result in two types of pain. The first is actual traumatic pain from the injury
itself, and the second pain is from the swelling that results. LLLT focuses first on the
lymphatic system which maintains the body’s fluid balance, while the laser light also
helps absorb the excess edema. LLLT thus provides relief in two ways.
MicroLight has FDA clearance for devices under both the “NHN” and “ILY”
classifications.The MicroLight ML830® was cleared by the FDA for treatment of
carpal tunnel syndrome. (See “About Us”). This clearance followed a double-blind
study on CTS that was conducted at General Motors.
Laser wavelengths between 820 nanometers (nm) and 840 nm have an extremely
low absorption rates in human tissue. This means that laser light penetrates deeply at
those frequencies.
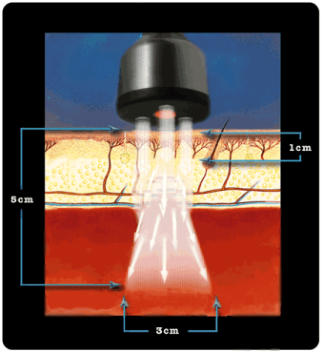
The ML830® is a GaAlA Laser that has wavelength of 830 nm with a power output of
90 mw. At this wavelength and power the ML830® Laser has a penetration of
approximately 5 cm with a 3 cm lateral spread.
It is not an accident that the Microlight Corporation of America chose & patented the
830nm technology for its ML830®. There are 30+ years of clinical studies that proved
the 830nm range is the optimal wave-length.
SPECS
GENERAL MOTORS DOUBLE BLIND STUDY
As further background, General Motors had serious problems with CTS among its workers.
GM responded by conducting a 36-week double-blind study, using the ML830® to see if non-
invasive conjunctive therapy would help. Among the 166 afflicted GM workers who entered the
program, those treated with the ML830® laser showed significant improvement in grip strength
and range of motion when compared to other GM workers who were treated with placebo
lasers. A prominent medical school in Houston conducted a similar double-blind study on CTS
in 1998 that showed a 70% improvement after conjunctive therapy using the ML830® among
those patients in the active group.
Picture of Laser:







Diodes
Three gallium aluminum arsenide laser diodes
Coherent
Beam travels in a straight line
Monochromatic
Form a single wavelength
Polarized
Beam is concentrated in one location or spot
Power
30 milliwatts per diode
Wavelength
830 nanometers
Energy
Delivers 3 joules of energy per 33 second treatment cycle


To order call 763-218-2075 Click for Pricing Guide
Financing available!
To order call 763-218-2075 Financing available!
Click for Pricing Guide


© Copyright Evolve Into A New You 2021 All rights reserved.

How it Works